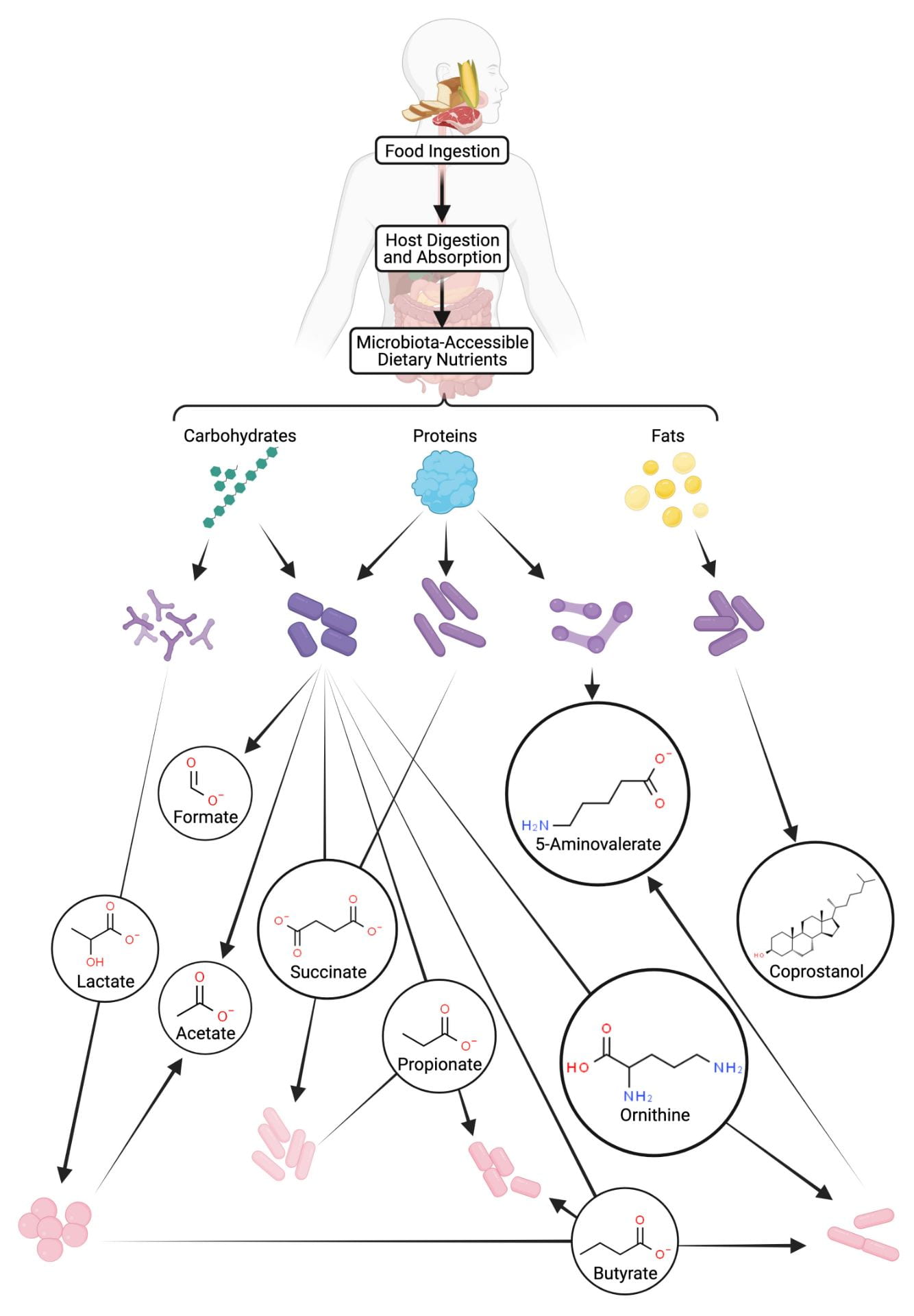
Different microbes within the human gut grow on distinct dietary components. The collective microbial activity produces a complex metabolic web that determines the concentration of host-modulating metabolites within the gastrointestinal tract. Illustration by Shuo Huang.
Hundreds of diverse microbes stably colonize the human gastrointestinal tract and makeup the gut microbiome. While only certain types of microbes are adapted for life in the gut, the presence and abundance of particular microbes within the microbiome varies greatly across the population. A particular microbial species may be abundant in one person’s gut microbiome, but absent from another’s. This type of compositional variability has been tied to numerous diseases (autoimmunity disorders, metabolic diseases, infectious diseases, brain function/development, etc.). Familiar ecological principles account for the number and diversity of microbes within the microbiome. In the same way that a gazelle and giraffe non-competitively depend upon distinct food sources within the savanna, microbes differentially specialize in energy extraction from diverse components of our diet (carbohydrates, protein, fats, etc.). Some microbes even feed on the waste products of other microbes, establishing something analogous to a food chain. The resulting web of feeding patterns converts dietary components into many drug-like small molecules called metabolites. Metabolites can enter the bloodstream and interact with host proteins, establishing one of the major mechanisms by which the microbiome impacts mammalian biology. Our lab is applying ecological and biochemical principles to deconstruct the microbiome’s microbial food web and relate the underlying metabolisms to the production or consumption of bioactive metabolites. Our long-term goal is to leverage insight about the microbiota’s metabolic functions to develop applications for diagnosing and treating disease.